Introduction: Pomalidomide is a third-generation immunomodulatory drug approved for relapsed and/or refractory Multiple Myeloma (RRMM). In a phase 3 OPTIMISMM trial, Pomalidomide, Bortezomib, and Dexamethasone demonstrated superior efficacy in patients with RRMM. PRIME study (CTRI/2019/10/021618) is testing Bortezomib, Pomalidomide and Dexamethasone combination in Newly Diagnosed Multiple Myeloma (NDMM). Initial data of 26 patients was presented as abstract in 63rd ASH Annual Meeting and Exposition. Current abstract is reporting the results of 46 patients with a longer follow up of this ongoing study.
Objectives: Primary objective is to determine the efficacy of VPD regimen by measuring Minimal Residual Disease (MRD) assessment by flow-cytometry after 9 cycles of VPD. Secondary objectives are to determine response rates, PFS and safety of the regimen
Methods: A prospective, single arm, phase II study from a tertiary care center. Both transplant eligible and ineligible patients with NDMM aged between 18-70 years as defined by the International Myeloma Working Group (IMWG) are being recruited in the study. The regimen consists of weekly Bortezomib 1.3mg/sq m, Pomalidomide 2 - 4mg once daily for 21days and Dexamethasone 20mg twice weekly, in a 28 days cycle for 9-12 cycles. Transplant patients planned for 9 cycles and non-transplant patients for 12 cycles of VPD. Eligible patients underwent High dose chemotherapy followed by Autologous HCT after 4-6 cycles of VPD. After Auto HCT 3-5 cycles of VPD given as consolidation therapy. After 9-12 cycles of VPD maintenance treatment given as per institutional practice.
A 2-tube 8-colour antibody panel was used for MRD analysis using Flowcytometry (BD FACS CANTO-II) and analysed on Kaluza version. 1.3 software. A valve of < 0.001% was considered as MRD negative.
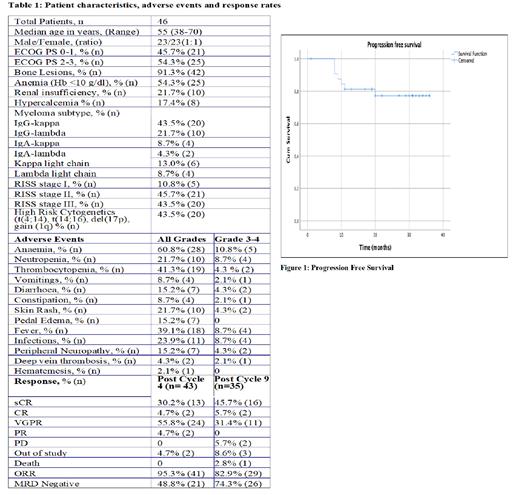
Results: In this ongoing study, out of the proposed 50 patients, 46 patients were enrolled between April 2020 to June 2023. Median follow up is 24.5 months. The median age was 55 years (38-70), and gender ratio 1:1. At disease presentation, bone lesions was the commonest (91.3%, n 42) finding. The most common myeloma subtype was IgG Kappa in 43.5% (n 20). High risk cytogenetics were seen in 43.5% (n 20) and 43.5% (n 20) of patients were in RISS stage III (Table 1).
Out of 46 patients 41 (89.1%) have completed 4 cycles and 30 (65.2%) patients have completed 9 cycles of VPD till last follow up (June, 2023). One patient had withdrawn consent, two patients were out of study due to increasing peripheral neuropathy (Grade 3). Five deaths occurred, 2 post cycle 8 and 3 after cycle 9. Four deaths were due to infections (COVID 19 and bacterial) and one due to progressive disease.
Five patients underwent auto HCT (between Cycle 4 to 6). No patient failed stem cell mobilization, 2 patients required plerixafor in addition to G-CSF for mobilization. Table-1 shows drug-induced toxicity and response rates. Hematological toxicities were the commonest followed by fever, infections and skin rash. Majority of nonhematological adverse events were grade 1 to 2 and protocol was well tolerated. Protocol adherence was 97.8% (45/46 patients).
After 4 cycles (n 43), 13 (30.2%) patients were in stringent complete response (sCR), 2(4.7%) in CR, 24 (55.8%) in very good partial response (VGPR) and 2 (4.7%) in partial response. After 9 cycles (n 35), 16 (45.7%) patients were in sCR, 2(5.7%) in CR and 11 (31.4%) in VGPR. Two (5.7%) patients had progressive disease (PD) and 3 patients were out of study due to consent withdrawn and toxicity. After 4 cycles of VPD 21 (48.8%) patients were MRD negative and MRD negative rate improved after 9 cycles to 74.3% (26 patients). Two years Progression Free survival (PFS) was 77.2% +/- 7.7% (Figure 1), disease free survival was 90.3% +/- 5.3% and overall survival (OS) was 83.1% +/- 7%.
Conclusion: Safety data from the PRIME study demonstrates that VPD regimen has a favorable tolerance profile in patients with NDMM and no clinically significant impact on stem cell mobilization or engraftment. Early efficacy signals are encouraging, and recruitment continued. VPD has shown improved depth of response (MRD negativity) and overall response rate. Early follow up results shows promising PFS and OS results. VPD may be a promising induction regimen instead of Dara VRD in resource constrain setting where Daratumumab is not readily accessible. Phase 3 randomized trials are the way forward to its clinical use in frontline setting.
OffLabel Disclosure:
Kumar:Dr Reddys Laboratories Ltd: Honoraria; Intas Oncology: Honoraria; Novartis Healthcare Private Ltd: Honoraria; Natco Pharma Ltd: Honoraria; Janssen oncology: Honoraria. Chattopadhyay:Dr Reddys Laboratories Ltd: Honoraria; Zydus Lifesciences Limited: Honoraria. Nair:Cipla Limited: Honoraria; Fresenius Kabi: Honoraria; Janssen oncology: Honoraria; Mylan: Honoraria; Novartis Healthcare Private Ltd: Honoraria; Dr Reddys Laboratories Ltd: Research Funding.
Drug - Pomalidomide: an immunomodulatory and antineoplastic drug. FDA approved for multiple myeloma in patients who have received at least 2 prior therapies. Purpose- Use in newly diagnosed Multiple Myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal